Projects in the Silverman Lab
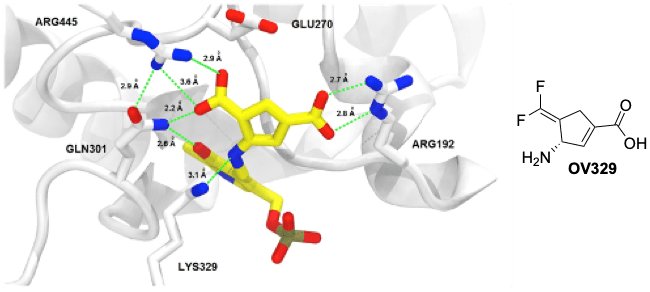
GABA AMINOTRANSFERASE (GABA-AT) INACTIVATORS
Gamma-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the central nervous system. Compounds that inhibit this enzyme raise brain GABA levels and are important in the treatment of epilepsy and addiction. We currently have a compound treating a boy with infantile spasms. Our lab is working to develop new mechanism-based inactivators of this enzyme and are studying their mechanisms of inactivation.
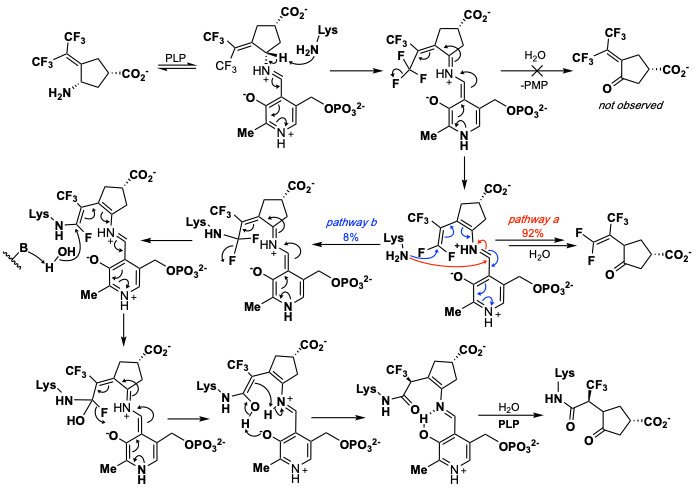
ORNITHINE AMINOTRANSFERASE (OAT) INACTIVATORS
Ornithine aminotransferase (OAT) has been found to be overexpressed in various cancer cells; we have reported the first compound that inactivates OAT and inhibits the growth of hepatocellular carcinoma in mice. We are making new OAT inactivators, studying their inactivation mechanisms, and investigating their ability to inhibit cancer growth in patient-derived xenografts.
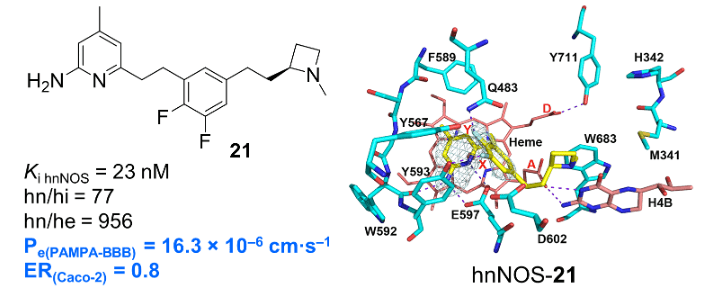
NITRIC OXIDE SYNTHASE (NOS) INHIBITORS
Nitric oxide is an important secondary messenger that is generated by nitric oxide synthase (NOS). This enzyme has three isoforms, one in the brain (nNOS), in macrophages (iNOS), and in endothelial cells (eNOS). Inhibition of nNOS could be important for the treatment of a number of neurodegenerative problems, such as Parkinson’s disease, Alzheimer’s disease, and cerebral palsy, but only if selective inhibition is attained. Our lab was the first to report dual-selective inhibitors of nNOS and have synthesized numerous new classes of compounds that are highly potent and selective for nNOS; we are improving upon their pharmacokinetics and blood-brain barrier penetration. We also have found nNOS inhibitors inhibit the growth of melanoma.
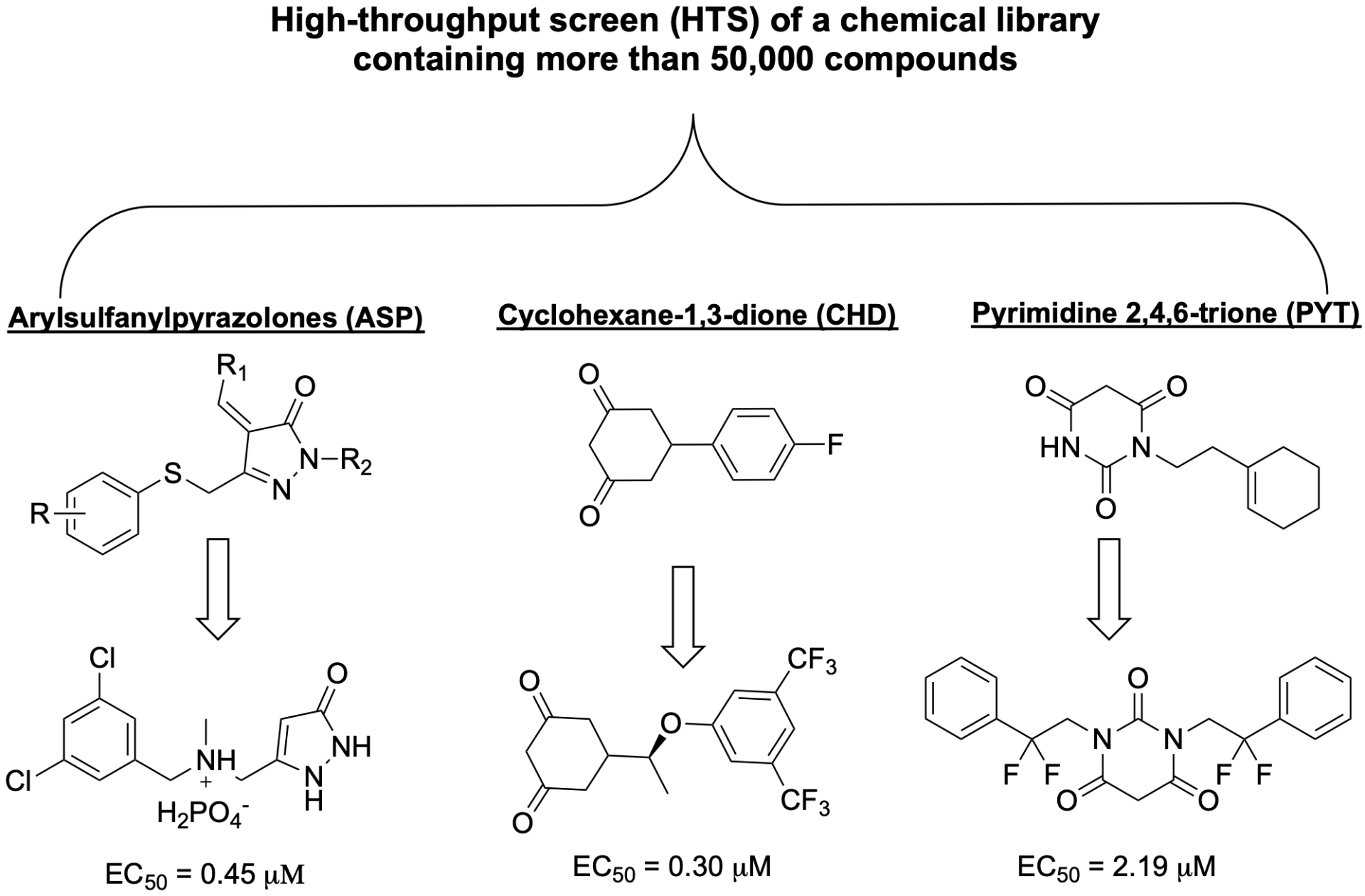
PROTEIN AGGREGATION INHIBITORS
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that affects motor neurons in the cortex and spinal cord, leading to rapid neuronal atrophy and death. An important hallmark of ALS (and all neurodegenerative diseases) is protein aggregation in the cytoplasm of degenerated neurons. The Silverman research group has identified three classes of compounds that inhibit protein aggregation and extend the life of the ALS mouse model. One analog eliminates degeneration of upper motor neurons (UMNs) diseased by both mutant SOD1 toxicity and TDP-43 pathology. Our current research goals are to determine the binding target of these compounds, to develop more potent protein aggregation inhibitors, and to expand to other neurodegenerative diseases.
